The advisory committee for the CDC met last week and decided on a common sense change regarding the Hep B vaccine. For the past thirty years, this shot has been given to every newborn, regardless of risk.
All of the committee members agreed that the committee lacks key data on the risks and benefits. The Food and Drug Administration (FDA) did not require randomized placebo-controlled clinical trials of the two Hepatitis B vaccines that it approved for the first day of life in the 1980s, according to FDA Acting Center for Drug Evaluation and Research Director Tracy Beth Høeg.
“The data that we used to approve the Hepatitis B vaccines … were based on studies that had a very short-term follow-up and no control group,” said Høeg. “We would never approve a vaccine with data like those today. … We are working with very low-level evidence here and we have very limited confidence when we say these vaccines are safe.”
There is also going to be a review of the entire vaccine schedule looking at information from other developed countries. Looking at the numbers, it’s about time.
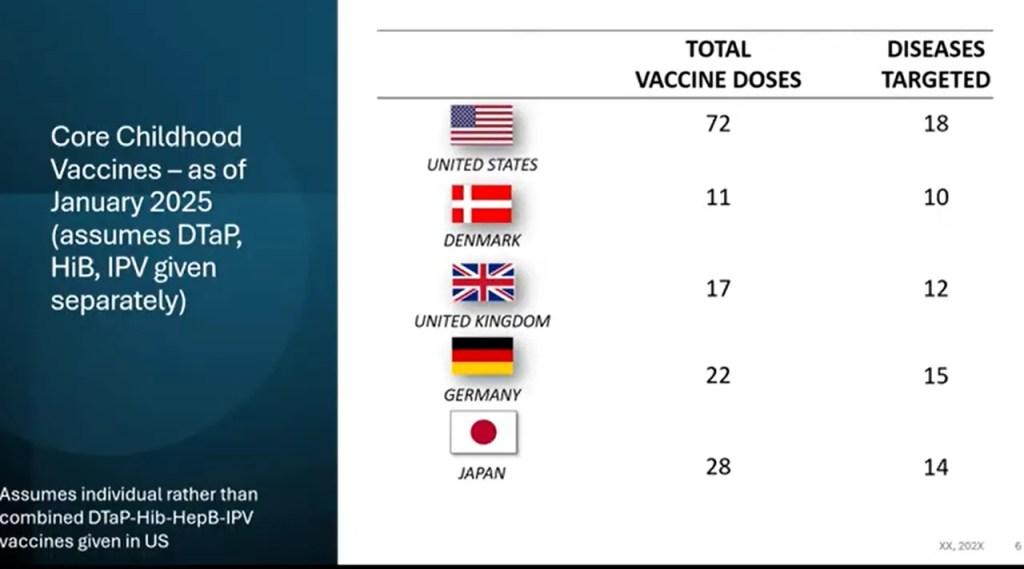
“In January 2025, the United States recommended vaccinating all children for 18 diseases, including COVID-19, making our country a high outlier in the number of vaccinations recommended for all children,” the memo will state. “Study is warranted to ensure that Americans are receiving the best, scientifically-supported medical advice in the world.”
Considering the fact that the vaccine schedule has been built on over the course of decades with no review and that the ‘tests’ were not true placebo testing that the public assumed was being done, it is about time that a comprehensive review happen.
Leave a Reply